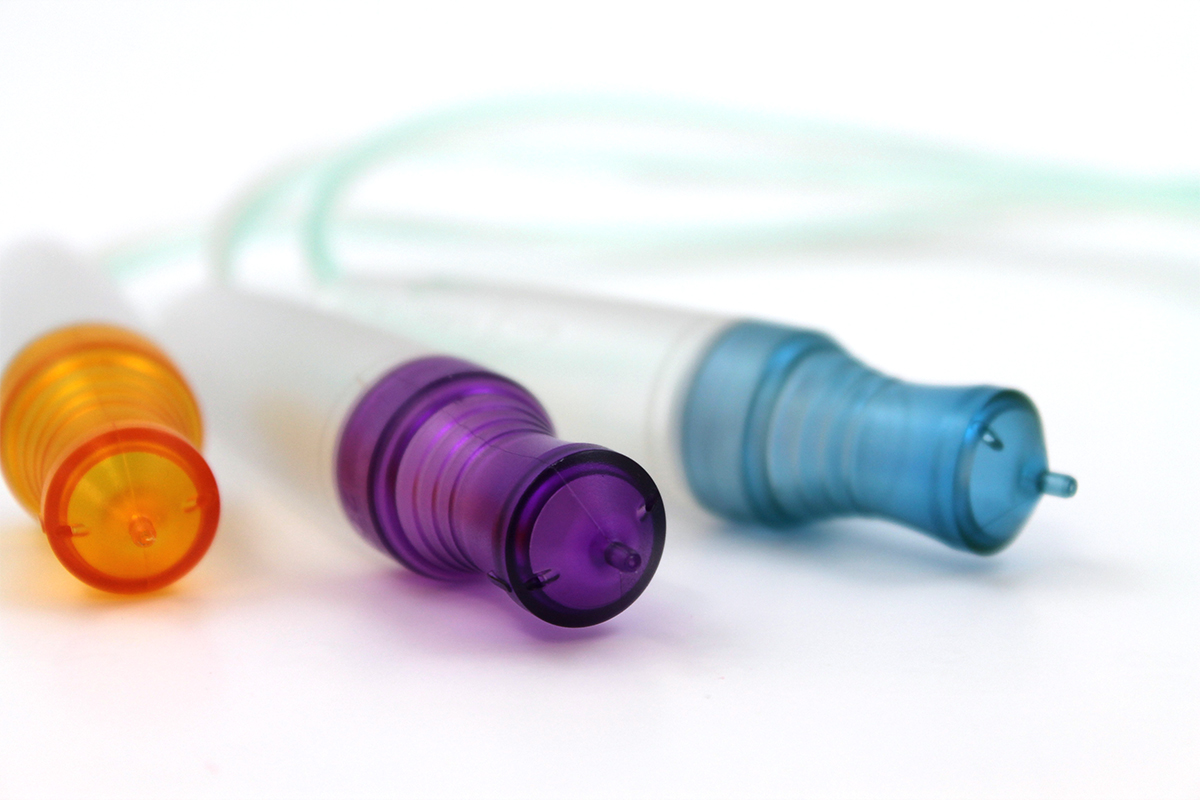
Traceable safety
The possibilities are endless: Granula produces a wide range of additives, including functional masterbatches with antimicrobial and aseptic properties, UV protection and, optionally, scratch resistance and resistance to disinfectants. If required, our colour masterbatches and compounds are developed for the medical market to meet the various requirements for medical devices. Our product range also includes effective products for achieving cycle times for injection moulding applications and laser marking additives for permanent marking. These products can also withstand sterilisation processes.
ISO 13485
To meet the requirements of the medical device industry, Granula implements the internationally recognised ISO 13485 (MedTech) quality system with objective proof of compliance.
All medical masterbatches and compounds are manufactured at Granula under IS0 13485:2003 certification. We guarantee full traceability of all batches, back to the raw materials used and the suppliers of these raw materials. All our masterbatches are also manufactured under strict controls with regard to traceability, consistency and change history.
MDR compliance in masterbatch production
The European Medical Device Regulation (MDR, EUR 2017/745) has been in force since May 2021 and replaces the previous Medical Device Directive (MDD). It sets out comprehensive requirements for the manufacture, approval and market surveillance of medical devices. Raw material suppliers such as masterbatch manufacturers are also affected if their products are intended for use in medical applications. Granula® masterbatch and colour masterbatch are manufactured in accordance with MDR for specific products with corresponding confirmations and material safety data sheets.
© Granula AG, last modification 08.09.2025

