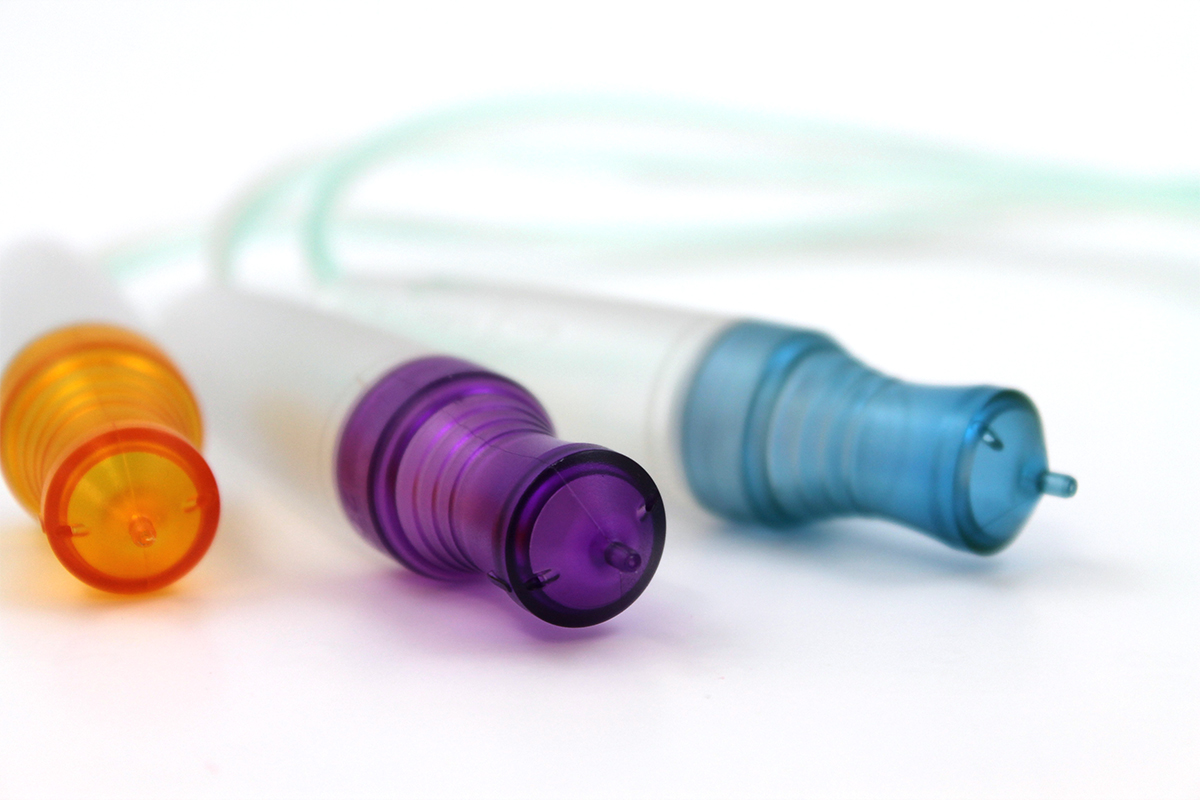
Masterbatches for the plastic production of medical components such as catheters, syringes, packaging materials or implants must meet the requirements of the Medical Device Regulation (MDR, EU 2017/745). The selection of compliant carrier materials, raw materials, ingredients and additives, as well as complete verification and documentation, are crucial for product safety. The MDR places high demands on all parties involved in the medical device value chain. For masterbatch manufacturers, this means an obligation to ensure maximum process reliability, comprehensive documentation, strictly compliance with substance limits and close cooperation with customers. Compliance with these requirements is not only necessary for regulatory reasons, but also helps to strengthen the company's position in international competition.
MDR compliance for masterbatch manufacturers

The European Medical Device Regulation (MDR, EU 2017/745) has been in force since May 2021 and replaces the previous Medical Device Directive (MDD). It sets out comprehensive requirements for the manufacture, approval and market surveillance of medical devices. Raw material suppliers such as masterbatch manufacturers are also affected if their products are intended for use in medical applications.
Materials and ingredients
The MDR regulates substances that can be used in medical devices, including carcinogenic, mutagenic and reprotoxic (CMR) substances, endocrine disruptors and nanomaterials. As a manufacturer of masterbatches, Granula® ensures that its products do not contain any prohibited or regulated substances.
Key requirements for MDR-compliant masterbatches
1. Technical documentation
Provision of safety data sheets, declarations of conformity and, where applicable, toxicological assessments. The technical documentation includes all material components for each individual production batch with their respective safety information.
2. Traceability
Each batch must be clearly documented to enable rapid traceability in the event of deviations.
3. Substance regulation
Restrictions on CMR substances and endocrine disruptors must be observed. Substitution options should be examined.
4. Cooperation with customers
Close cooperation with medical device manufacturers is necessary in order to efficiently support their approval process in accordance with MDR.